Cabinet Notes: The Minister of Health gave an update on the successes of Covid-19 treatment in Antigua and Barbuda.
The number of active cases remains in single digits at 4.
The Cabinet advises that the population is to continue to exercise vigilance, wearing masks, practicing social-distancing, and sanitizing hands as frequently as reasonable.
There is to be no relaxation of the vigilance that has kept the numbers low, the Cabinet agreed.
The Cabinet was informed that the PAHO list of vaccines, which will be made available to the participating COVACS members, does not include the Pfizer vaccine nor does it include the Moderna vaccine; the Oxford vaccine is included.
Antigua and Barbuda is a participating COVACS member.
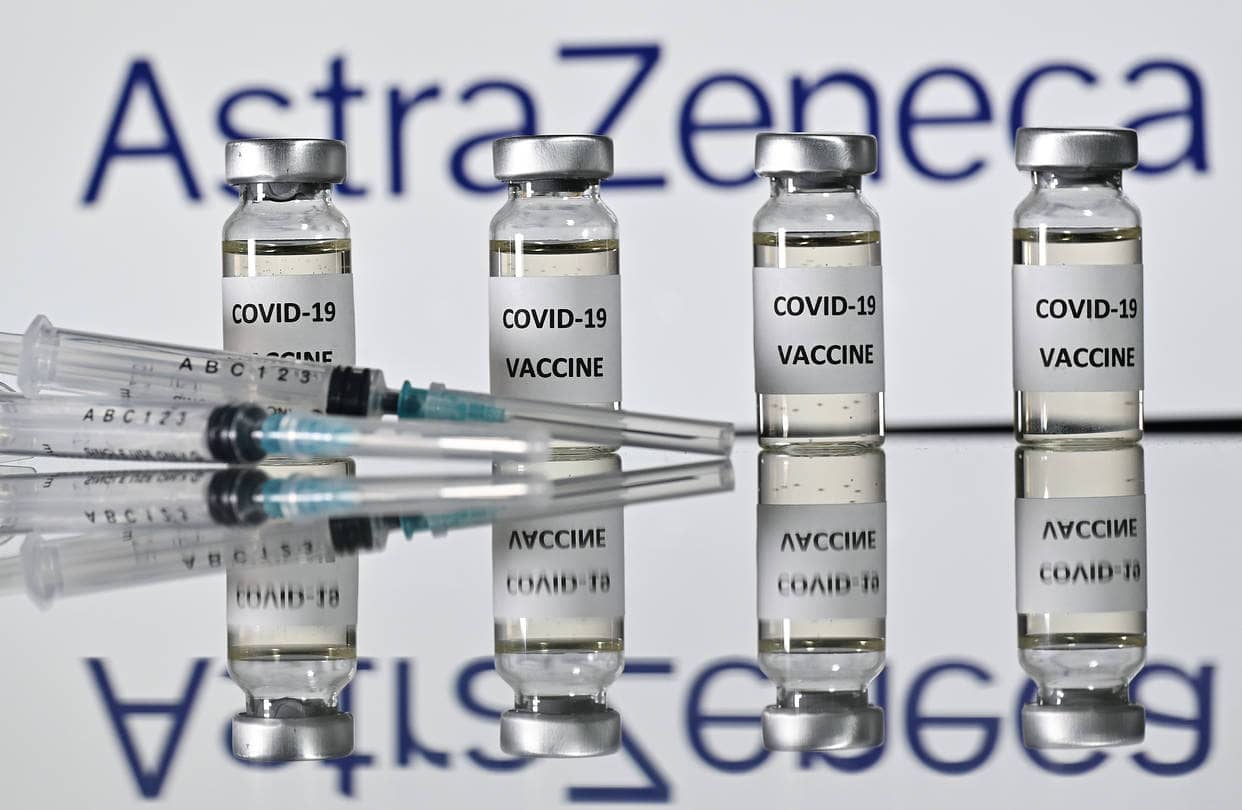
AstraZeneca said on Monday that its Covid-19 vaccine candidate, which is being developed with the University of Oxford, was found to be as much as 90% effective in preventing infections, according to data from a late-stage clinical trial in the U.K. and Brazil.
Interim analysis from the Phase 3 vaccine trial found that the experimental shot is 90% effective when administered as a half dose followed by a full dose one month later. Another dosing regimen showed 62% efficacy when given as two full doses at least one month apart.
The combined analysis from both dosing regimens showed an average efficacy of 70.4%, said the University of Oxford and drugmaker AstraZeneca.
“These findings show that we have an effective vaccine that will save many lives,” said Andrew Pollard, chief investigator of the Oxford Vaccine Trial, in a statement. “Excitingly, we’ve found that one of our dosing regimens may be around 90% effective and if this dosing regime is used, more people could be vaccinated with planned vaccine supply,” he added.
AstraZeneca’s news comes days after drugmaker Pfizer and its German partner BioNTech requested emergency-use authorization of their Covid-19 vaccine candidate, shown to be 95% effective, in the U.S.
Moderna has said that its vaccine candidate is 94.5% effective and that the biotech company is also expected to apply for emergency-use authorization shortly.
However, AstraZeneca’s vaccine can be stored at normal refrigeration temperatures, not needing the supercool storage the Pfizer vaccine requires.
“It is hugely significant from a logistics point of view that storage can be achieved at a normal refrigeration temperature of around -3 degrees. This makes it much more scalable and as such easier to deliver on a global scale,” said Michael Hewson, chief market analyst at CMC Markets U.K.
AstraZeneca said there were no serious safety events related to the vaccine and it was well tolerated across both dosing regimens. Late-stage clinical trials of the vaccine are continuing in the U.S.
The Anglo-Swedish drugmaker said it is making “rapid progress” in manufacturing, with a capacity of up to 3 billion doses of the vaccine in 2021 on a rolling basis, pending regulatory approval.
AstraZeneca will immediately prepare regulatory submission of the data to authorities around the world that have a framework in place for conditional or early approval. It will also seek an emergency-use listing from the World Health Organization to speed up availability in low-income countries.
In parallel, the University of Oxford is submitting the full analysis of the interim results for publication in a peer-reviewed journal.
Looking ahead. AstraZeneca and the University of Oxford’s Covid-19 vaccine trial failed to trigger a major rally in equities, with the 70% efficacy result perhaps disappointing in comparison to the 95% results from Pfizer and Moderna.
But the results of the trial mark a fresh breakthrough in combating Covid-19. which has killed almost 1.4 million people worldwide and rocked the economy. “Astra’s vaccine is cheaper, easier to deliver around the world and much easier to produce in large quantities than the Pfizer or Moderna versions, so it will likely play a big role global immunization,” said Neil Wilson, chief market analyst at Markets.com.
AstraZeneca has promised to distribute the vaccine at cost. Susannah Streeter, analyst at Hargreaves Lansdown, said that although the breakthrough won’t boost profits in the short term, it has got long-term potential and will add to expertise in vaccines that AstraZeneca has lacked in the past.
Advertise with the mоѕt vіѕіtеd nеwѕ ѕіtе іn Antigua!
We offer fully customizable and flexible digital marketing packages.
Contact us at [email protected]

















Bad reaction to Oxford COVID-19 vaccine likely ‘life-threatening’: Expert
A serious illness suffered by an Oxford University coronavirus vaccine trial participant was most likely “life-threatening”, a bioethics researcher says. But that doesn’t mean the clinical trials will be scrapped.
AstraZeneca and Oxford University have suspended the late-stage study after a participant in Britain experienced what has been called a serious adverse reaction. Dr. Xavier Symons, a postdoctoral research fellow at Australian Catholic University’s Plunkett Centre for Ethics, said it was “concerning” the illness was described as a “serious adverse event”. “The term ‘serious adverse event’ means life-threatening illness and admission to the ICU,” Dr. Symons told The New Daily.
[https://thenewdaily.com.au/news/coronavirus/2020/09/09/vaccine-trial-covid-adverse-reaction/]
1) Can someone explain why should anyone take a vaccine for an ‘alleged virus’ with a 99.99% survival rate?
2) Why does anyone believe the virus actually exists because it has NEVER been isolated and the only knowledge we have about it comes from ‘computer modeling’?
3) Why are airlines talking about ‘vaccine travel documents’ in order to travel by air?
4) Why are we required to wear masks when there has never been any peer reviewed studies proving that they even work against a virus and in spite the fact that thousands of doctors have said they are causing ‘self harm’ and that we shouldn’t be wearing them?
5) Why are government leaders telling their people to listen to globalist elites who are self professed eugenicists’?
@ I have some questions
Many Pastors preach about hell at a sermon, alot of people get fearful and said they are saved because of fear of going to hell instead of turning for love of God .
The media and the wicked people in high places scared the people about deadly virus and tell them their only hope is vaccine, what is happening people are moving on fear. All of the goverment is in it together all of them follow suit with the same measures they have in place.
When the natural herbalist share their knowledge on plants and herbs to fight viruses that do exist, the pharmaceutical industries rejects the information and said their is no scientific evidence but a vaccine and virus that is new they believe in.
Man has become gods forsaking and condemning nature for wealth as they destroy the health of others with their pretentious love for mankind acting like they care for the lives of the sick
Here comes Great Britain once again!…and history repeats itself without us black people learning our lesson.
Are you suggesting Antigua makes it own vaccine? Sounds like you’re a little sore about the U.K. helping out buddy?
Gaston say he taking the 1st one.
Where are Nigel Christian’s killers?
What is to become of those refrigerators so hastily acquired by the government?
Comments are closed.